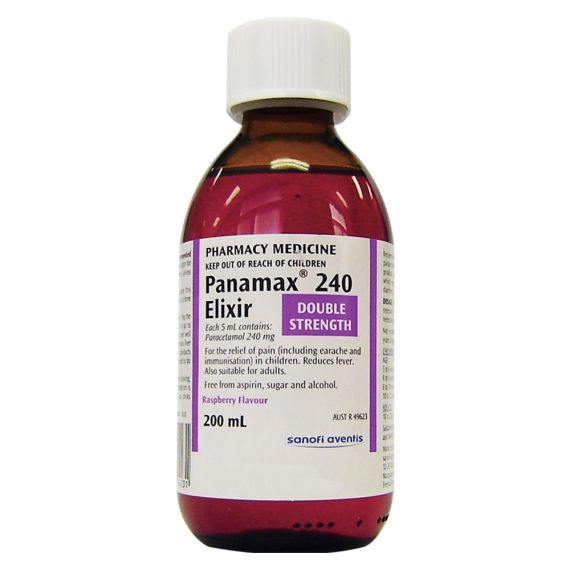
Panamax 240 Elixir 200ml
Relief of pain and discomfort in arthritis, headache, muscular and neuralgic conditions. Reduces fever. Panamax is useful as an analgesic for patients with dyspepsia, ulcers or gout.
$
5
.89
Relief of pain and discomfort in arthritis, headache, muscular and neuralgic conditions.
Reduces fever. Panamax is useful as an analgesic for patients with dyspepsia, ulcers or
gout.
Each 5 mL contains paracetamol 240 mg.
The inactive ingredients are: macrogol 1500, allura red AC CI 16035, propylene glycol,
glycerol, saccharin sodium, sorbitol solution, benzoic acid, potassium sorbate, raspberry
flavour, imitation candied sugar and purified water.
240 Elixir
Administer in water or fruit juice if necessary.
Children
5 to 6 years: (18a€"20 kg) 6 mL;
6 to 8 years: (20a€"25 kg) 6 to 8 mL;
8 to 10 years: (25a€"32 kg) 8 to 10 mL;
10 to 12 years: (32a€"41 kg) 10 to 12 mL.
Adults
10 to 20 mL (maximum 80 mL per day)
If necessary repeat 4 to 6 hourly up to 4 times in 24 hours.
Panamax 240 Elixir is not recommended for children under 5 years of age.
CONTRAINDICATIONS
Panamax is contraindicated in patients who are hypersensitive to paracetamol or to any
other component of the Panamax formulations. It must not be used in patients with known
glucose-6-phosphate-dehydrogenase deficiency or pre-existing respiratory depression.
Panamax must not be used in patients with impaired liver function.
PRECAUTIONS
Panamax should not be administered to patients with hepatic or renal dysfunction (see
CONTRAINDICATIONS). This medication may be dangerous when used in large amounts
or for long periods. Hepatotoxicity may occur with paracetamol even at therapeutic doses,
after short treatment duration and in patients without pre-existing liver dysfunction.
Hepatotoxicity may develop following as little as 10 to 15g of paracetamol and hepatic failure
is known to occur occasionally with the long term use of paracetamol.
Patients with known analgesic intolerance or known bronchial asthma must only use
Panamax after having consulted a physician (hypersensitivity reactions including
bronchospasm possible).
Caution is advised in patients with underlying sensitivity to aspirin and/or to non-steroidal
anti-inflammatory drugs (NSAIDs).*
Severe cutaneous adverse reactions (SCARs): Life threatening cutaneous reactions
Stevens-Johnson syndrome (SJS), and Toxic epidermal necrolysis (TEN) have been
reported with the use of paracetamol. Patients should be advised of the signs and symptoms
and monitored closely for skin reactions. If symptoms or signs of SJS and TEN (e.g.
progressive skin rash often with blisters or mucosal lesions) occur, patients should stop
paracetamol treatment immediately and seek medical advice.
Use in pregnancy
Category A - Drugs which have been taken by a large number of pregnant women and
women of childbearing age without any proven increase in the frequency of malformations or
other direct or indirect harmful effects on the foetus having been observed.
Paracetamol can cross the placenta; however, no teratogenic effects have been observed in
rats or mice, after doses of up to 250 mg/kg.
A woman in the third trimester of pregnancy ingested 22.5 g paracetamol. Early treatment
with oral acetylcysteine resulted in good outcome for both mother and foetus.
Use in lactation
Paracetamol is excreted in breast milk. The amount available for ingestion by the infant has
been reported variously as less than 0.1% of a single 500 mg dose and as 0.04 to 0.23% of
a single 650 mg dose. Maternal ingestion of paracetamol in usual analgesic doses does not
appear to present a risk to the nursing infant.Panamax PI
GLUv3 Piv8 18 Feb 14
INTERACTIONS WITH OTHER MEDICINES
Paracetamol may increase the risk of bleeding in patients taking warfarin and other
antivitamin K. Anticoagulant dosage may require reduction and patients should be monitored
for appropriate coagulation and bleeding complications.
Paracetamol absorption is increased by drugs which increase gastric emptying e.g.
metoclopramide and decreased by drugs which decrease gastric emptying e.g.
propantheline, antidepressants with anticholinergic properties, narcotic analgesics.
Paracetamol may increase chloramphenicol concentrations by slowing down excretion,
entailing the risk of increased toxicity. The risk of paracetamol toxicity may be increased in
patients receiving other potentially hepatotoxic drugs or drugs that induce liver microsomal
enzymes, such as antiepileptics (such as phenobarbital, phenytoin, carbamazepine,
topiramate), barbiturates, hypnotics, rifampicin and alcohol.
Paracetamol excretion may be affected and plasma concentrations altered when given
probenecid.
Colestyramine reduces the absorption of paracetamol if given within 1 hour of paracetamol.
Co-administration of flucloxacillin with paracetamol may lead to metabolic acidosis,
particularly in patients presenting risk factors of glutathione depletion, such as sepsis,
malnutrition or chronic alcoholism.
When used concurrently with zidovudine, an increased tendency for neutropenia may develop.
Combination of Panamax and zidovudine should be avoided.
ADVERSE REACTIONS
Reports of adverse reactions are rare. Although the following reactions have been reported:
dyspepsia, sweating, erythema, urticaria, anaphylactic shock, angioneurotic oedema,
difficulty breathing, drop in blood pressure, nausea, allergic reactions such as skin rashes,
hypersensitivity reactions and haematological reactions, including thrombocytopenia,
leukopenia, neutropenia agranulocytosis and pancytopenia. Bronchospasm may be triggered
in patients having a tendency of analgesic asthma. Toxic epidermal necrolysis (TEN),
Stevens-Johnson syndrome (SJS), acute generalised exanthematous pustulosis, fixed drug
eruption (see PRECAUTIONS) and cytolytic hepatitis, which may lead to acute hepatic
failure, have also been reported. Overdosage with paracetamol if left untreated can result in
severe, sometimes fatal liver damage and rarely, acute renal tubular necrosis.
Haemolytic anaemia in patients with underlying glucose 6-phosphate-dehydrogenase
deficiency has been reported. Kounis syndrome and bronchospasm have also been
reported.